Author information
Gertrude-Emilia Costin*
Download
Institute for In Vitro Sciences, Inc. (IIVS), 30 W Watkins Mill Road #100, Gaithersburg, MD 20878, USA
Abstract
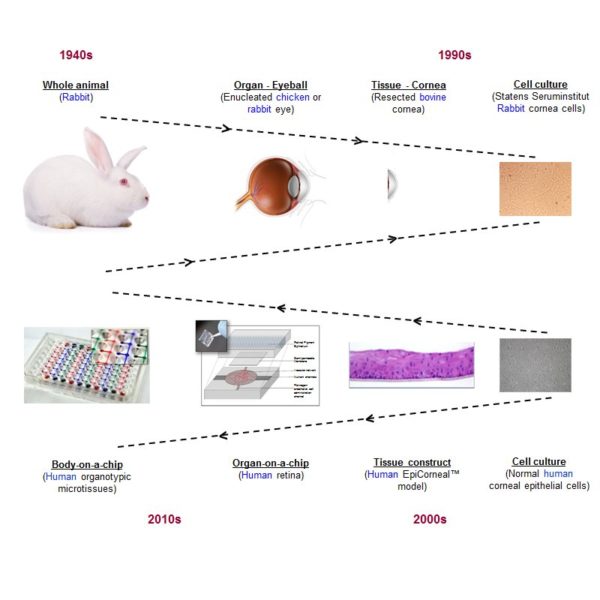
Toxicology testing platforms represent the basis of the human health risk assessment process that determines whether a material or product may induce harm to humans upon exposure. Historically, safety assessment of raw ingredients or finished formulations has been performed using animal-based test methods (in vivo) that provide whole organism responses to toxicants. Due to the large number of products launched by industry continuously, modern toxicology shifted in recent years towards the use of novel, fast and reliable alternative methods, ranging from in silico to in chemico or in vitro, of which some are validated for regulatory purposes. The manuscript also addresses emerging technologies in the form of “organ/body-on-a-chip” platforms which announce to be instrumental in allowing alternative systems to in vivo models to assess systemic toxic effects induced by chemicals.
(Received 23 February, 2017; accepted 18 March, 2017)
Keywords
predictive toxicology; in vitro methods; in silico methods; organ-on-a-chip