Author information
Saravanakkumar Chennappan1, Tanja Schulze1, Ion Cristian Cirstea1, 2, *
Download
1Leibniz Institute on Aging, Beutenberg Str. 11, 07745 Jena, Germany
2Institute of Comparative Molecular Endocrinology (CME), Ulm University, Helmholtz Str. 8/1, 89081 Ulm, Germany
2Institute of Comparative Molecular Endocrinology (CME), Ulm University, Helmholtz Str. 8/1, 89081 Ulm, Germany
Abstract
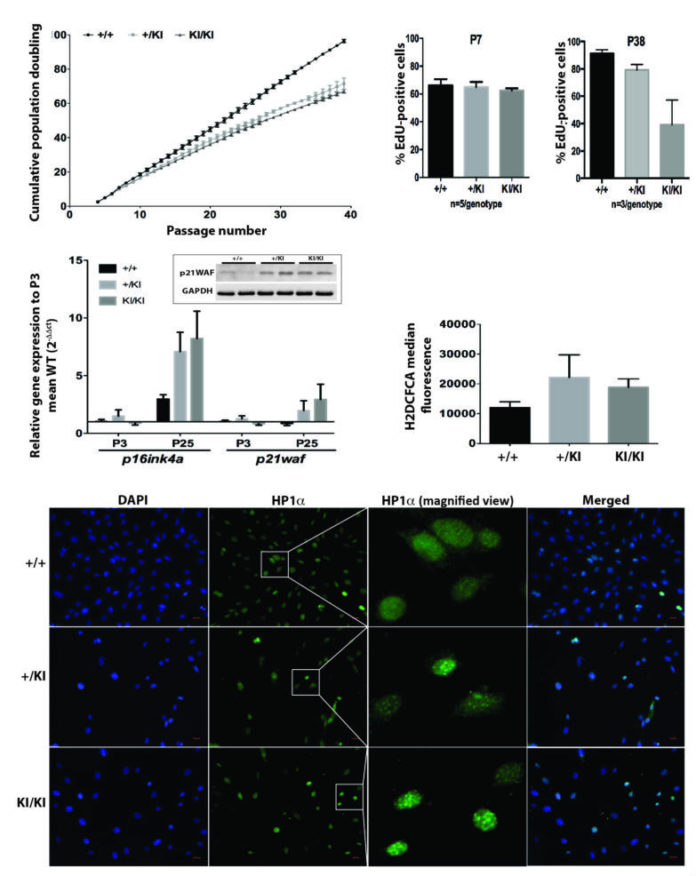
Oncogenic H-Ras germline mutations are associated with Costello syndrome (CS), a developmental disorder characterised by the failure to thrive, skin and musculoskeletal abnormalities, cardiomyopathy, and susceptibility to tumour development. Despite harbouring H-Ras oncogenic mutations, in both patients and a CS mouse model, the tumour incidence is very low. Using CS mouse primary fibroblasts, we identified that unlike the lack of tumorigenic transformation, the MAPK and Akt pathways display subtle changes in their activation kinetics. In vivo, these subtle changes might not be sufficient to induce malignant transformation, but may well be responsible for inducing CS pathologies. Moreover, we identified an oncogene-induced senescence-like phenotype, and we consider it as a potential mechanism in order to block tumour development in CS. The induction of oncogene-induced senescence-like phenotype can ultimately affect proliferative cells (e.g., progenitor cells) and impair tissue homeostasis during embryonic development and throughout the life span of CS patients.
(Received 9 March, 2017; accepted 23 April, 2017)
Keywords
endogenous, H-Ras, rasopathies, Costello syndrome, oncogene-induced senescence, tumour suppression, pathologies