Author information
Jayhun Lee1, 2 and Tudorita Tumbar1*
Download
1Department of Molecular Biology and Genetics, Cornell University, Ithaca, NY 14853, USA
2Present address: Morgridge Institute for Research, University of Wisconsin-Madison, Madison, Wisconsin 53715, USA
2Present address: Morgridge Institute for Research, University of Wisconsin-Madison, Madison, Wisconsin 53715, USA
Abstract
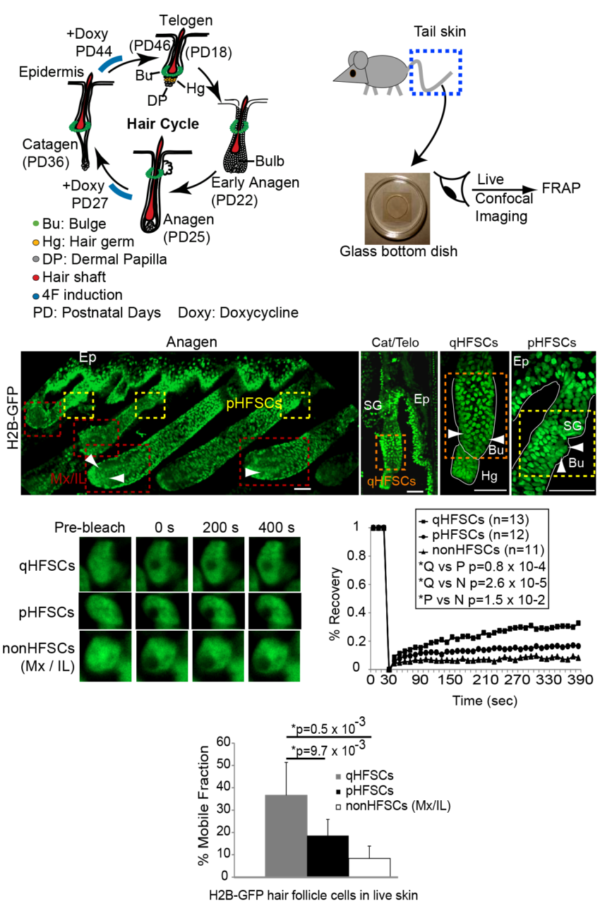
Cellular plasticity for fate acquisition is associated with distinct chromatin states, which include histone modi cations, dynamic association of chromatin factors with the DNA, and global chromatin compaction and nuclear organization. While embryonic stem cell (ESC) plasticity in vitro and its link with chromatin states have been characterized in depth, little is known about tissue stem cell plasticity in vivo, during adult tissue homeostasis. Recently, we reported a distinct globally low level of histone H3 K4/9/27me3 in mouse hair follicle stem cells (HFSCs) during quiescence. is occurred at the stage preceding fate acquisition, when HFSC fate plasticity must be at its highest. is hypomethylated state was required for proper skin homeostasis and timely hair cycle. Here, we show both in the live tissue and in cell culture that at quiescence HFSCs have higher exchange rates for core histone H2B when compared with proliferative or di erentiated cells. is denoted a hyperdynamic chromatin state, which was previously associated with high cell fate plasticity in ESCs. Moreover, we nd that quiescent HFSCs display a higher propensity for de- di erentiation in response to Yamanaka’s reprogramming factors in vivo. ese results further support our recent model in which HFSCs render their chromatin into a speci c state at quiescence, which is attuned to higher cell fate plasticity.
(Received 4 March, 2017; accepted 10 May, 2017)